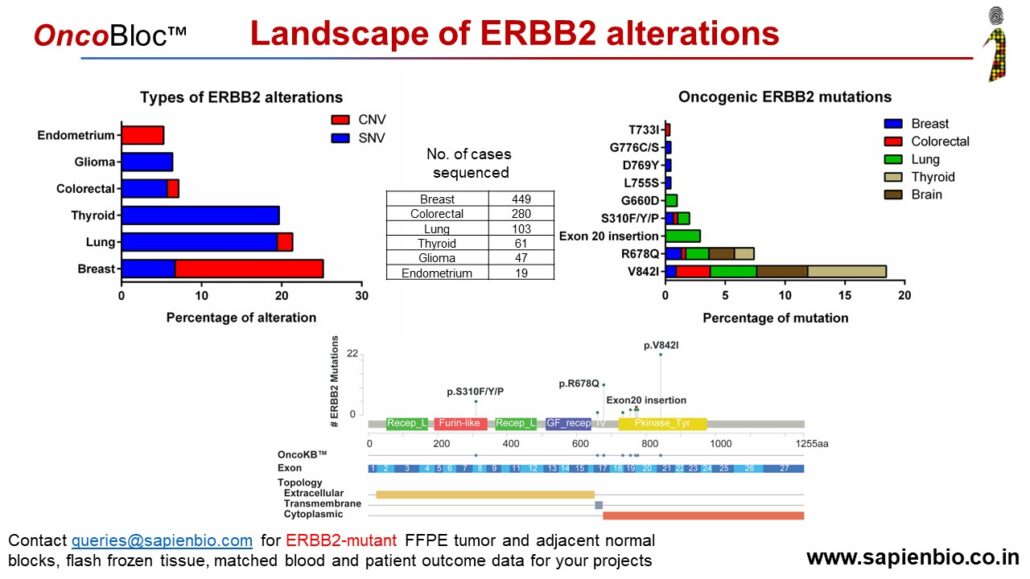
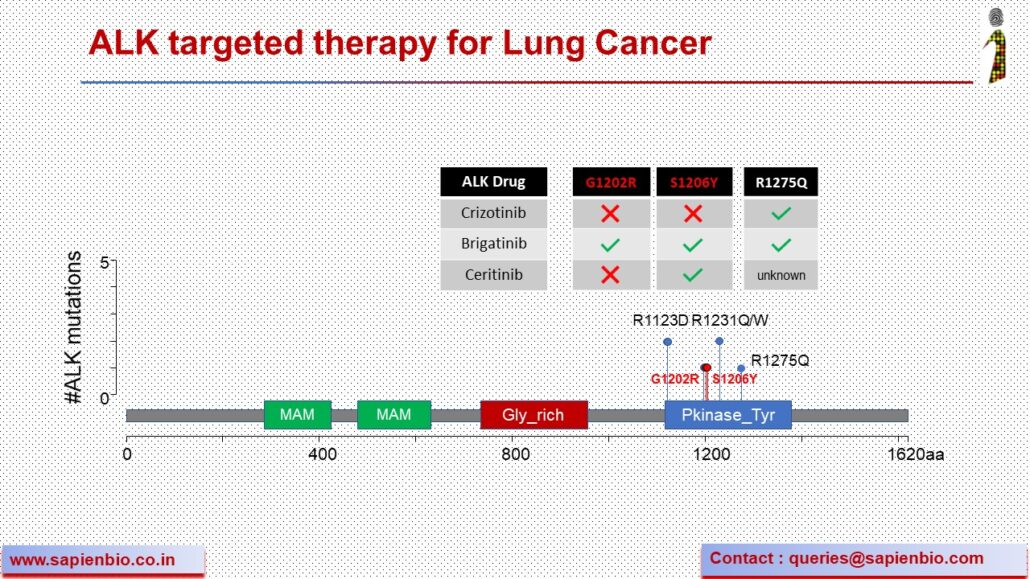
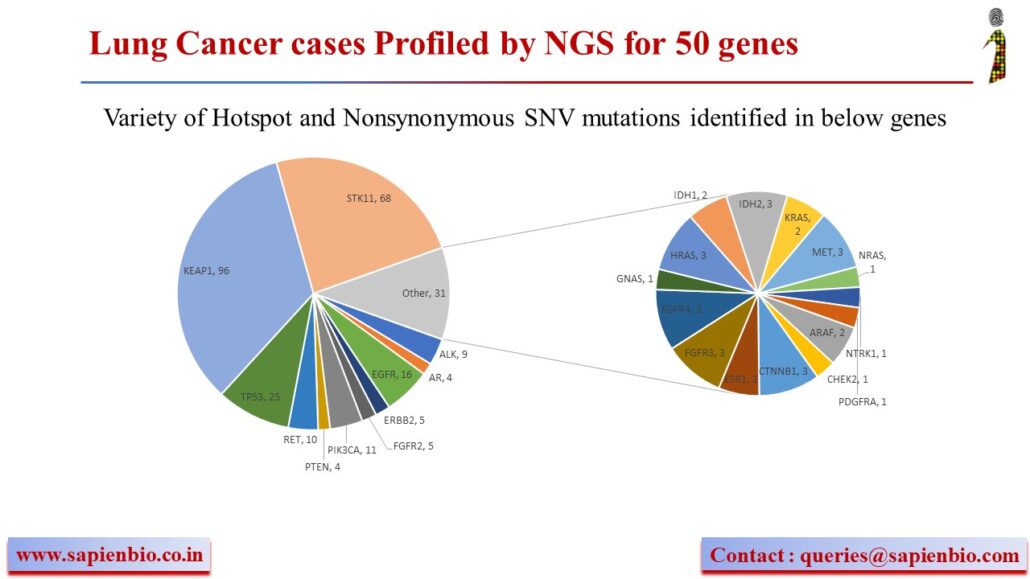
HER2 Oncogenic mutations and amplifications are common across many Indian solid tumors
Until recently, HER2 (or ErbB2) targeting drugs were only approved for HER2-amplified breast & gastric cancers. FDA has now granted accelerated approval for trastuzumab or Enhertu to treat lung cancer patients bearing activating HER2 mutations. We analyzed our data of ~1000 solid cancer cases generated using the OncomineTM Dx NGS panel to determine the pattern of ERBB2 amplification vs. mutations. We see that in some cancers, amplifications are more common (breast, gastric, endometrial cancers) but in others, activating mutations are more common (colorectal, lung, thyroid and gliomas) with the most common mutation being V842I in Indian samples. Molecular insights gained from such NGS analysis can hopefully improve precision medicine by expanding the use of approved HER2-targeting drugs for colorectal, endometrial, gliomas, thyroid and other cancer patients that are likely to benefit from them.




Recent Comments