Some driver RET mutations are not observed in Indian cancers
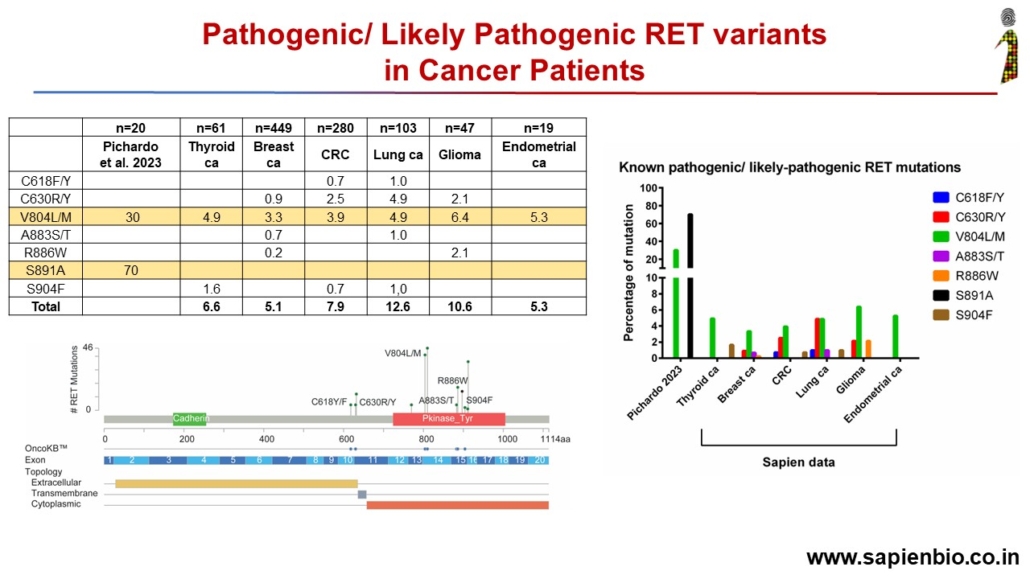
The impact of two driver mutations of RET gene, V804M/L and S891A, on Thyroid cancer was described recently by Pichardo et al in JAMA Otolaryngology, 2023. In a population screening, 75 people were identified to have 2 Pathogenic or Likely Pathogenic (P/LP) germline variants of the RET gene. 20 out of these 75 patients chose to undergo prophylactic thyroidectomy even though they did not have any symptoms of cancer. After surgery, pathological analysis of their tissue detected the growth of medullary or papillary thyroid cancer (PTC) in 12 and 2 patients respectively, i.e., 70% of patients with these 2 mutations had been harboring cancer unknowingly!
We examined the presence of these 2 RET variants in Sapien’s 61 Indian thyroid cancer samples profiled in the OncoMineDx panel by ThermoFisher. P/LP mutations were seen in 4 cases, all below the age of 40. Three cases were PTCs and 1 Follicular, with an overall percentage of 6%. The variants detected were V804M/L (3 cases) and C618Y (1 case, Follicular). No case of S891A mutation was detected.
We also checked for RET mutations in our genetically profiled lung cancers (103 cases) where 28 cases had SNVs with 5 cases of V804M, and 1 case had a fusion. No case of S891A was observed. Similarly, among 449 breast cancers, 280 CRCs, 47 gliomas and few cases each of endometrium, urinary bladder and prostate cancers that have been genetically profiled, many cases of V804M/L and other P/LP RET mutations were observed but none of S891A.

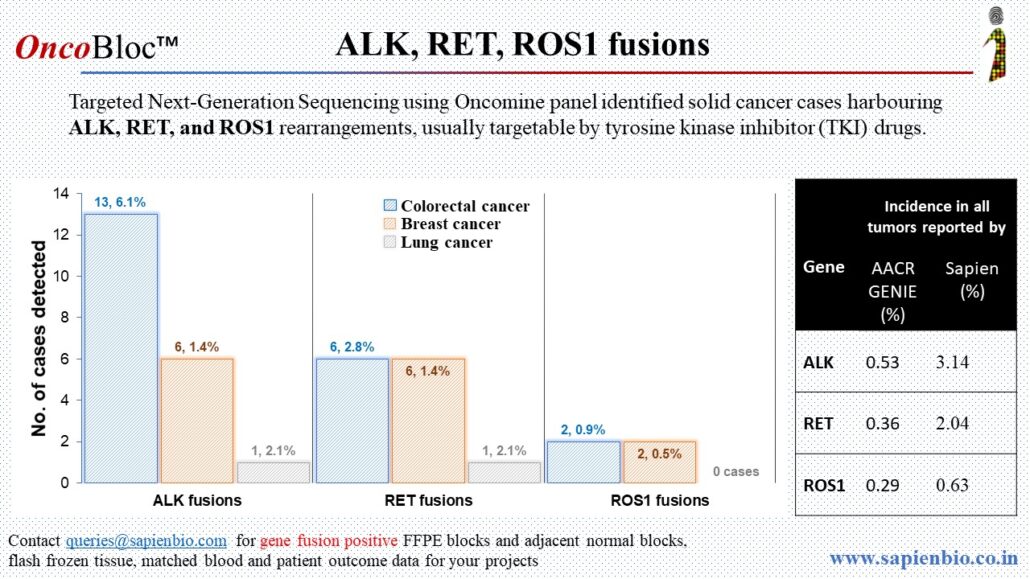
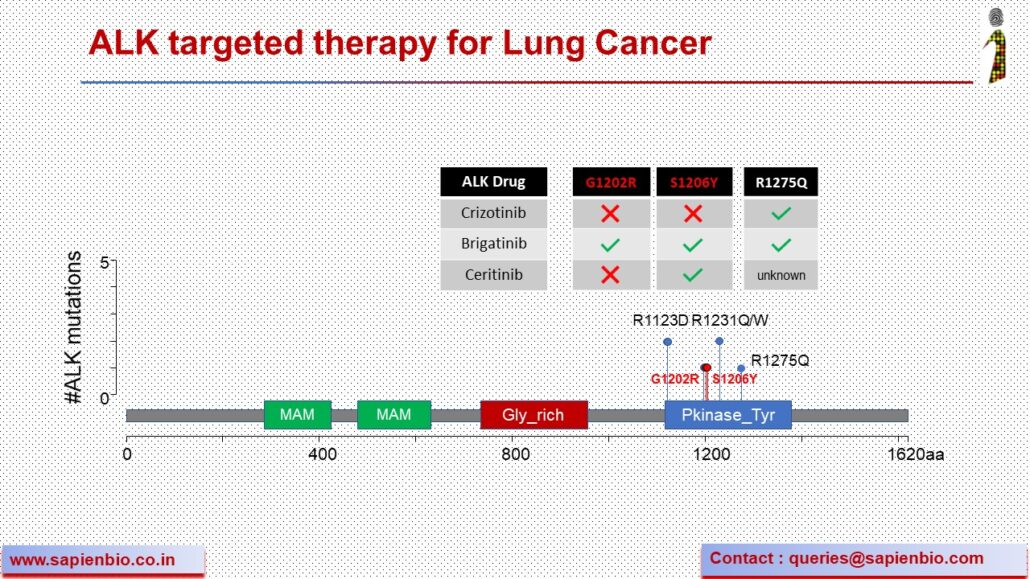
Recent Comments