ALK targeted therapy for lung cancer: Integration of NGS genome profiling in management of ALK-mutated NSCLCs in India
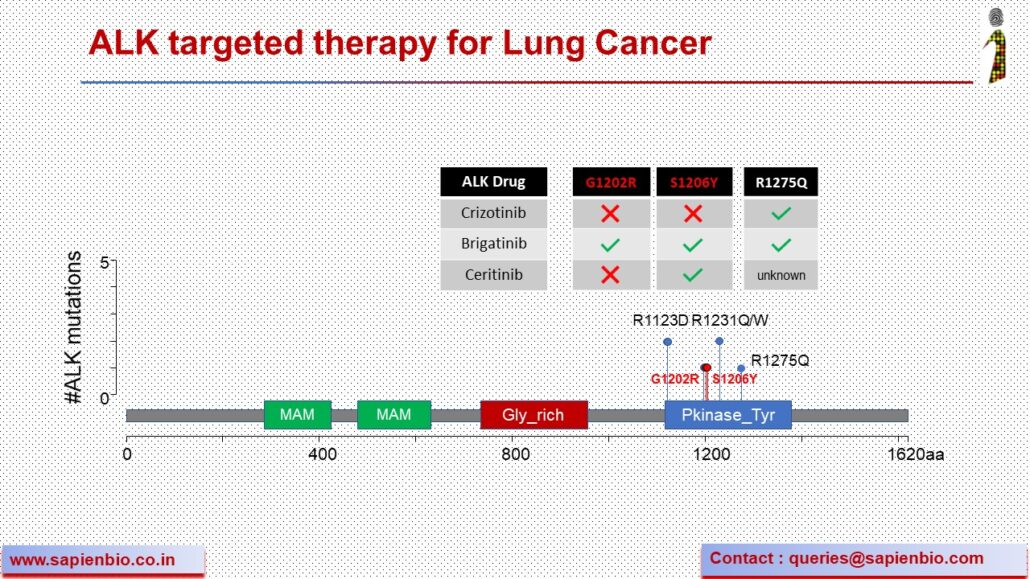
The NGS genotyping of our NSCLC cases using ThermoFisher’s Oncomine panel identified 8 genetic variants in the ALK gene in 9 cases (16.67%), some of which, such as G1202R and S1206Y surprisingly confer resistance to #Crizotinib treatment, but demonstrate sensitivity to second-generation ALK inhibitors such as #Brigatinib and #Ceritinib, which are currently approved for the treatment of metastatic lung cancers (Sullivan I et al., Ther Adv Med Oncol 2016).
Integration of NGS genetic profiling of tumour samples could play a beneficial role in the management of ALK mutated NSCLCs in India by helping identify the best targeted therapy among Brigatinib, Ceritinib and Crizotinib upfront, based on the mutational profile.
https://www.cancer.org/cancer/lung-cancer/treating-non-small-cell/targeted-therapies.html

Recent Comments