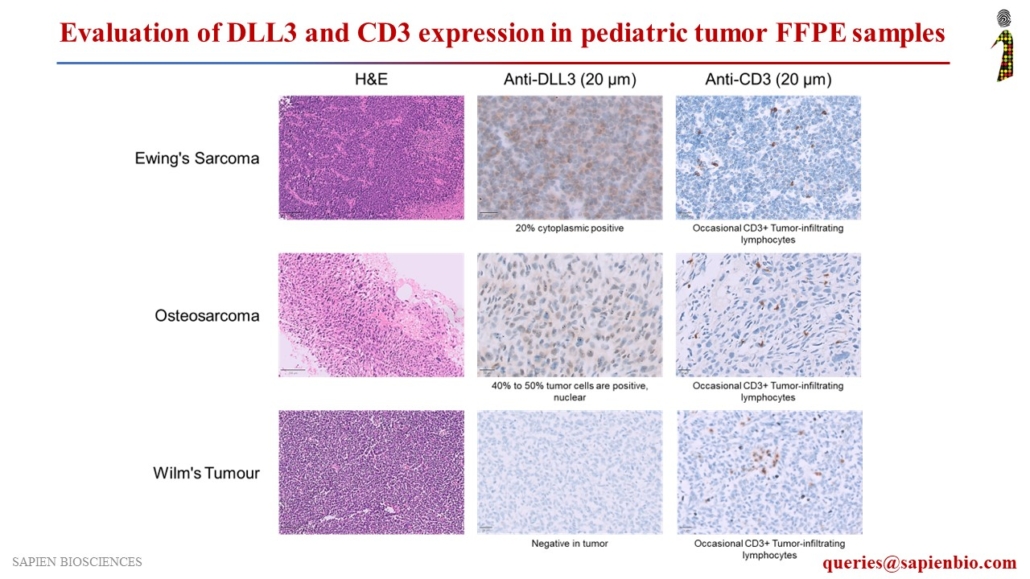
Assessment of DLL3 and CD3 Expression in Pediatric Tumor FFPE Samples for Potential DLL3-Targeted Immunotherapy
Delta-like ligand 3 (DLL3), a Notch inhibitory ligand, is a promising therapeutic target that is upregulated in Small Cell Lung Cancers (SCLC) and Neuroendocrine carcinomas but is not detectable in normal adult tissues. Various DLL3-specific therapies are under clinical development, including ADC rovalpituzumab tesirine (#Abbvie), bispecific TCE molecule AMG 757 (#Amgen), and CAR-T therapy AMG 119 (#Amgen), for the treatment of SCLC and neuroendocrine carcinomas.
We evaluated DLL3 expression in several tumor types, along with CD3, a marker for T-cell infiltration in tumors, to assess their potential for DLL3-targeted immunotherapeutic agents. DLL3 showed positive staining in neuroblastoma and pediatric tumors, such as Ewing’s sarcoma, osteosarcoma, rhabdoid tumors, Hodgkin’s lymphoma, rhabdomyosarcoma, acute myeloid leukemia (AML), and acute lymphoblastic leukemia (ALL).

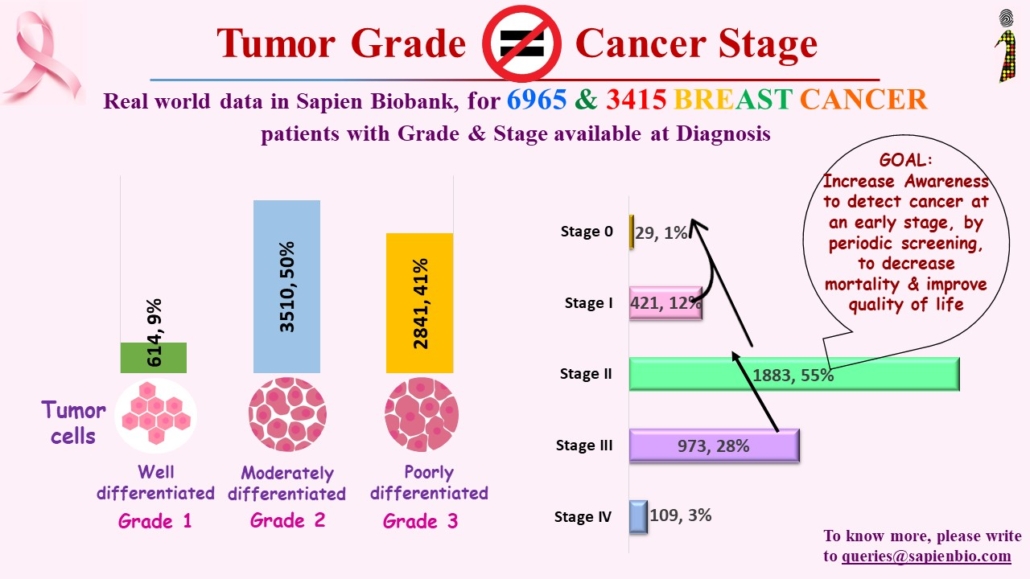
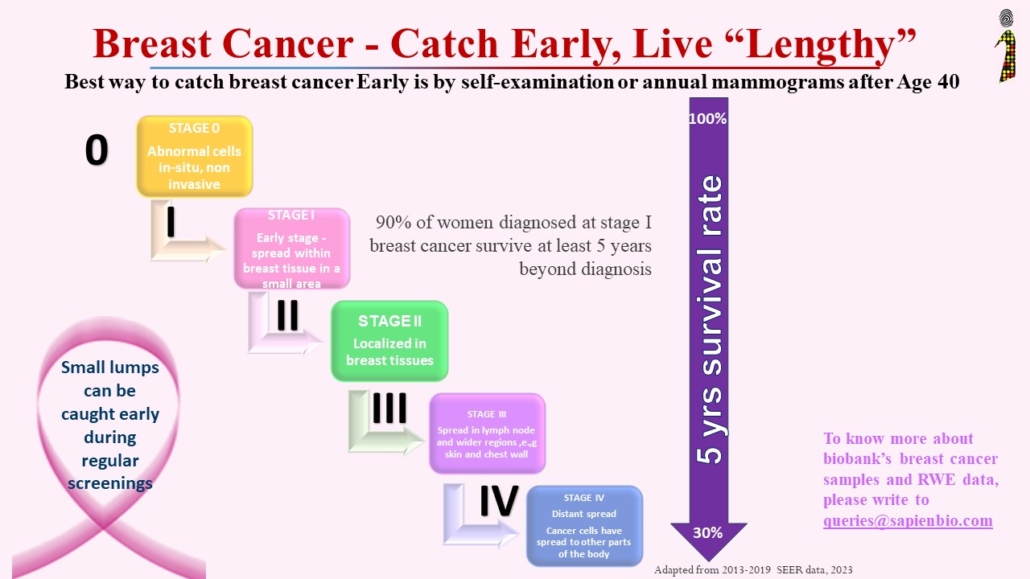
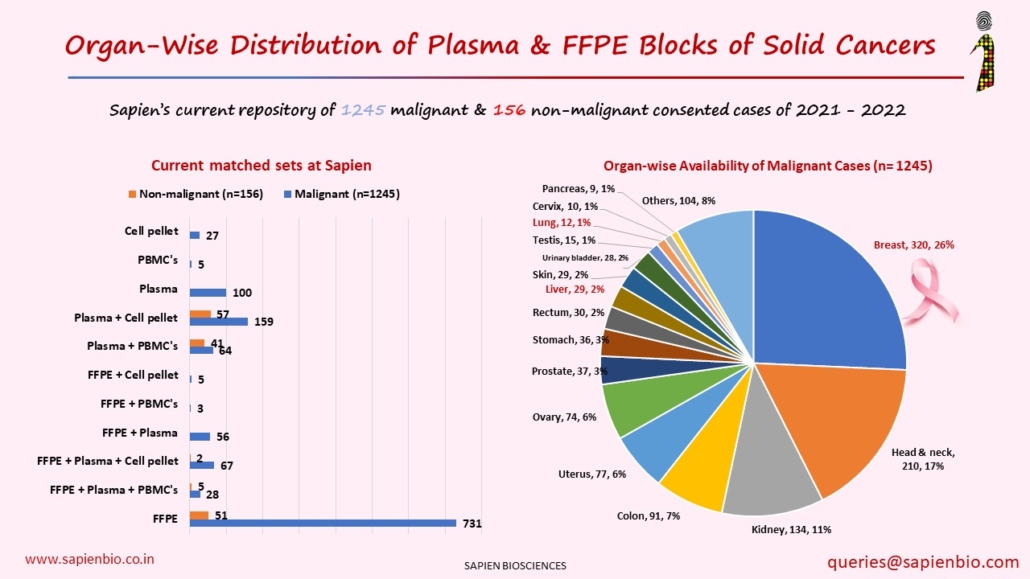

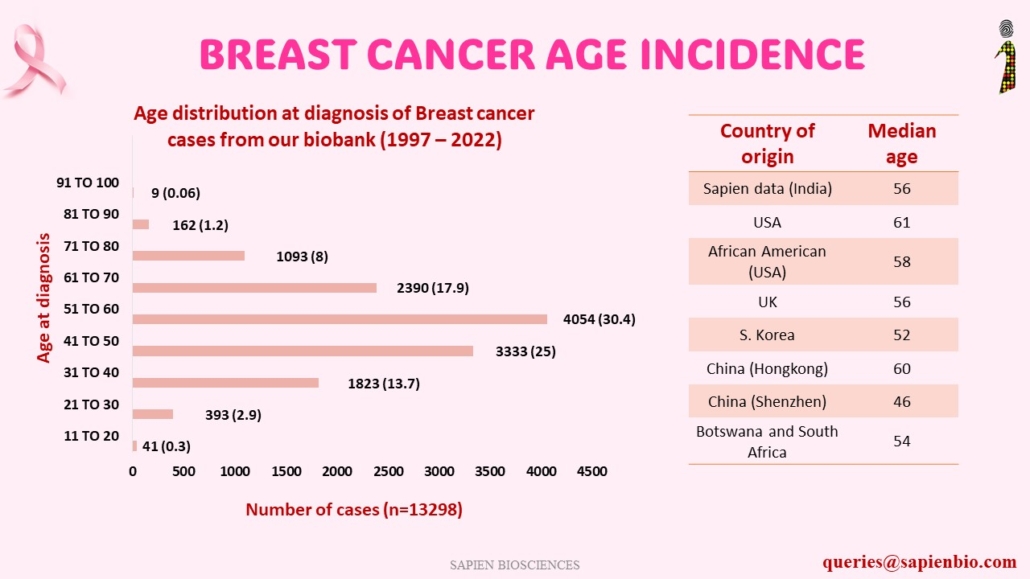
Recent Comments